April 16th-17th
Empirical and Molecular Formula
What is an Empirical and Molecular Formula?
An empirical formula is the simplest ratio of elements in a compound. For example, the empirical formula for ethane is CH3. This formula cannot be reduced any further, since it is already in its simplest ratio.
A molecular formula is the actual number of each atom in the molecule in a compound. For example, the molecular formula for ethane is C2H6. This formula tells us the actual number of each atom (which in this case is 2 carbon atoms and 6 hydrogen atoms in each molecule).
What is the Difference?
Many compounds have the same empirical formulas, but different molecular formulas. For example, benzene (C6H6) and acetylene (C2H2) both have an empirical formula of CH, but their properties are very different:
| |
Benzene |
Acetylene |
| Formula |
C6H6
|
C2H2 |
| State |
Liquid at room temp. |
Gas at room temp. |
| Chemical Property |
Flammable |
Highly combustible |
| Uses |
Used in gasoline |
Used in welding torches |
|
 |
 |
Even though they have the same empirical formula, it is their molecular formula that tells us how many atoms of each element there are and determines the properties of each compound. Here is a video that helps show the difference between empirical and molecular formula.
Calculations Involving Empirical and Molecular Formula
Calculating Empirical Formula
You can calculate the empirical formula for a compound using this song:
Empirical Formula Song (to the tune of “Happy Birthday”)
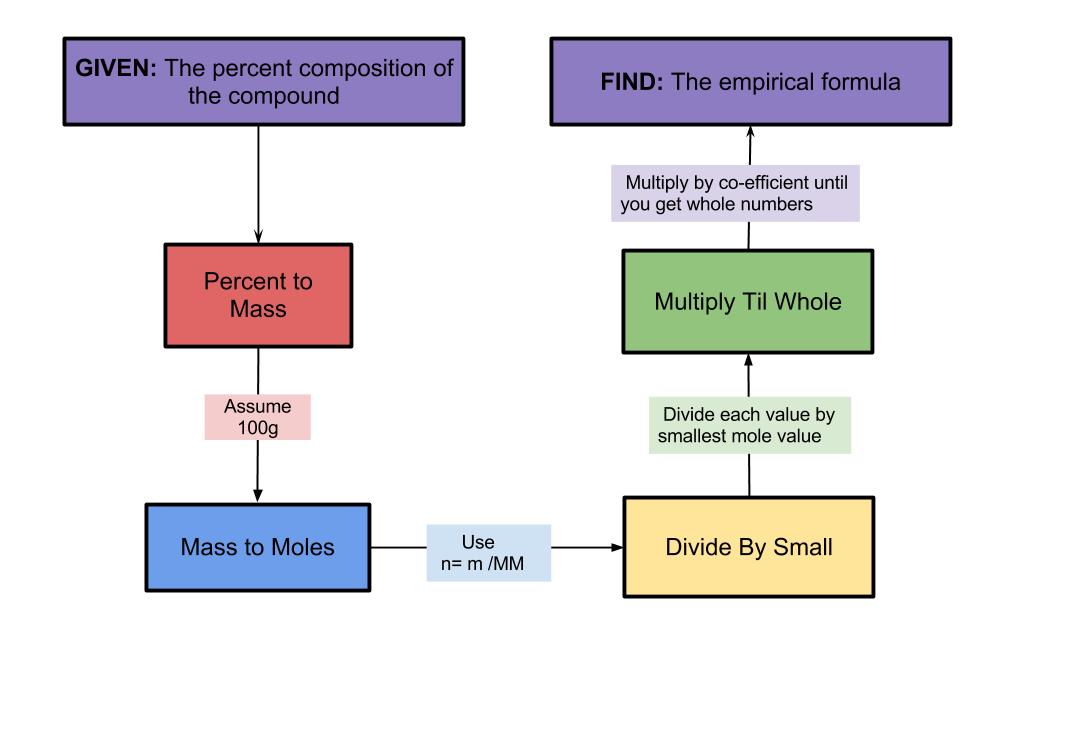
1. Percent to Mass
2. Mass to Moles
3. Divide By Small
4. Multiply Til Whole
Example #1: What is the empirical formula for a compound that contains 80.04% carbon and the remainder is hydrogen?
Step 1: Percent to Mass
To convert from percent to mass, we assume that there is 100 grams. The ratios would not change no matter how many grams we assumed there was, but 100 is an easy number to use. By doing this, the mass will equal the same number as the percent, but in grams.
mc= 80.04 g
mH= 19.96 g (100 g- 80.04 g)
Step 2: Mass to Moles
To convert from the mass to moles, you use the formula n=m/MM, where n=moles in mol, m=mass in g and MM=molar mass in g/mol. The mass is the number calculated in the previous step and the molar mass is found on the periodic table. You only need to write the formula once in your answer.
nc= mc nH= 19.96g
MMc 1.01g/mol
= 80.04g =19.76238 mol
12.01g/mol
= 6.66445 mol
Step 3: Divide By Small
To determine the ratio of the elements, you set the moles you calculated in a ratio and divide all the numbers by the smallest number.
nc: nH= 6.66445 mol: 19.76238 mol
÷ 6.66445= 1: 2.97
Step 4: Multiply Til Whole
When elements form compounds, the atoms combine in simple whole number ratios. This means that the subscript of the formula must be whole numbers and not decimals. For example, you cannot have C3.5H7 because you cannot have half of an atom. So, if you don’t get all whole numbers in step 3, you will need to multiply by a co-efficient to get a whole number using this chart:
| If it ends in... |
Multiply by... |
| 0.33 |
3 |
| 0.5 |
2 |
| 0.25 |
4 |
| 0.75 |
4 |
If none of these work, simply try multiplying by 2, then 3, then 4, then 5, etc until you get a whole number.
****NOTE: If you are within 0.05 of a whole number (the decimal value is between 0.01 and 0.05 or 0.95 and 0.99) then you can round to the nearest integer.****
In this example, 2.97 is within 0.05 of 3.00, since it is only 0.03 away, so you can round to 3.00 and you do not have to do this step.
Final Answer: To determine the empirical formula, you use the numbers you calculated in step 3 or 4 and use them as the subscript for the elements in the formula. So, for this question, the empirical formula is CH3.
Example #2: What is the empirical formula for a compound that contains 82.6% carbon and 17.4% hydrogen?
1) Percent to Mass
mc= 82.6 g
mH= 17.4 g
2) Mass to Moles
nc= mc nH= 17.4 g
MMc 1.01 g/mol
= 82.6 g =17.22776 mol
12.01 g/mol
= 6.87760 mol
3) Divide By Small
nc: nH= 6.87760 mol: 17.22776 mol
= 1: 2.5
4) Multiply til Whole
nc: nH = 1: 2.5
= 2 : 5
Therefore, the empirical formula is C2H5.
Calculating Molecular Formula
Ionic compounds are always in their empirical formula, since you reduce them to their simplest ratios when doing nomenclature. For example, if you get Mg2O2, you reduce it to MgO, which is its empirical formula. However, most covalent compounds have different empirical and molecular formulas, so you must know how to calculate the molecular formula.
There is a factor that exists between a compound’s empirical and molecular formula. For example, between C5H4O (the empirical formula) and C15H12O3 (the molecular formula), there is a factor of 3. When you compare their molar masses, the same factor exists. For example, the molar mass of C5H4O is 80.09 g/mol, which is 3 times less than the molar mass of C15H12O3, which is 240.28 g/mol. You can use this relationship to calculate the molecular formula.

Example #3: What is the molecular formula for a compound that contains 82.6% carbon and 17.4% hydrogen? The molar mass of the compound is 116.28 g/mol.
Step 1: Calculate the Empirical Formula
Although it does not ask for the empirical formula, you must calculate it to determine the molecular formula. Remember- percent to mass, mass to moles, divide by small and multiply til whole.
Since we already calculated the empirical formula in the previous question, we can skip this step.
Step 2: Compare the empirical and molecular formula and find the factor.
Using the empirical formula you calculated, find the molar mass. Then compare it to the molar mass of the molecular formula given in the question and divide the molecular molar mass by the empirical molar mass.
MMemp. = 2 (12.01g/mol) + 5 (1.01g/mol)
=29.07 g/mol
MMemp. = 29.07 g/mol <---------- x 4 ------------> MMmol. = 116.28 g/mol (from the question)
So, the molecular formula is 4 times greater than the empirical.
Step 3: Multiply the subscript in the empirical formula by the factor.
Therefore, the molecular formula of this compound is C8H20.
Here are a video with more examples of empirical and molecular formula calculations.
If that isn't working for you, try watching this video. It is a song about empirical and molecular formula.
Here is the link for another video that summarizes everything discussed on this wiki page. By request of the video owner, embedding is disabled, but you can click on the link below to access the video.
http://youtu.be/eHR9saDmeYk
In Summary
- The empirical formula is the simplest ratio of elements in a compound and the molecular formula is the actual number of each atom in the molecule.
- Compounds can have the same empirical formula, but different molecular formulas and very different properties.
- To calculate the empirical formula, use the song- percent to mass, mass to moles, divide by small and multiply til whole.
- To calculate molecular formula, find the factor between the molar masses of the empirical and molecular formula and multiply the subscript by that factor.
Practice
For some practice on calculating the empirical and molecular formula, try these online quizzes by clicking on the pictures below:


If you have any questions about empirical and molecular formula, just leave a comment.
Comments (0)
You don't have permission to comment on this page.